- A-
- A
- A+
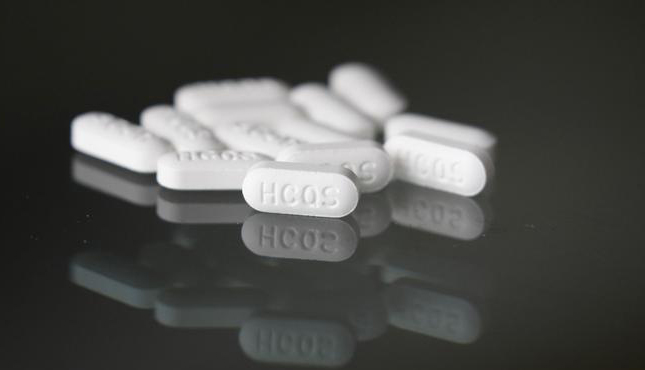
US revokes emergency use of malaria drugs for COVID-19
The United States has ended its authorization of hydroxychloroquine and chloroquine for treating COVID-19 patients, citing reports of heart complications.
The US Food and Drug Administration (FDA) on Monday revoked its emergency authorization for two malaria drugs as potential COVID-19 treatments.
The drugs hydroxychloroquine and chloroquine — touted by President Donald Trump as a treatment for the coronavirus — are unlikely to be effective, the FDA said amid growing evidence they don't work and could cause deadly side effects.
The regulator said the drugs pose a greater risk to patients than any potential benefits.
Citing reports of heart complications, the FDA said the decades-old drugs' unproven benefits "do not outweigh the known and potential risks.''
Similar News
Links



 Elm TV
Elm TV
 Photo
Photo
 Video
Video